Coulomb Force
Dans la pratique le coulomb est. Remember too that charges of the same.

What Is Coulomb S Law Laws Of Electrostatics With Example Basic Electrical Engineering Electric Flux Law
Although the law was known earlier it was first published in 1785 by French physicist.

. The TraPPE-UA2 united atom version 2 force field is a more accurate united-atom force field. Induction 4 Electric Field 5 Electric Field cont 6 Electric Flux. While applying Coulombs Law to find out the force between two point charges we have to be careful of the following remarks.
F k e q₁q₂r². In other words a force can cause an object with mass to change its velocity. If class II force fields are selected in the input command file additional cross terms are computed as part of the force field.
It is convenient to label one of these charges q as a test charge and call Q a source charge. The above equation is the vector form of Coulombs Law. Visualize the electrostatic force that two charges exert on each other.
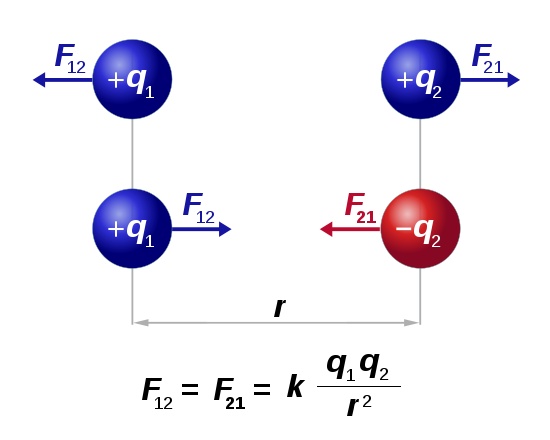
And K e is the Coulombs constant with a value of 9987 10 9 Nm 2C 2. Hence the law and the associated formula was named after him. COULOMBS LAW The separation of the centers of the spheres is 2R so the distance we use in Coulombs law is r 2R 259 1015 m 118 1014 m so from Eq.
Coulombs law also known as Coulombs inverse-square law is a law of physics that defines the amount of force between two stationary electrically charged particles known as the electrostatic forceCoulombs law was discovered by Charles-Augustin de Coulomb in 1785. As we develop the theory more source charges will be added. Charge 1 - Measured in Coulomb - The Charge 1 is a fundamental property of forms of matter that exhibit electrostatic attraction or repulsion in the presence of other matter.
It was named after the French physicist Charles-Augustin de Coulomb 17361806 who introduced Coulombs law. N is the SI unit of forceIt is named after Sir Isaac Newton because of his work on classical mechanicsA newton is how much force is required to make a mass of one kilogram accelerate at a rate of one metre per second squared. Coulombs inverse-square law or simply Coulombs law is an experimental law of physics that quantifies the amount of force between two stationary electrically charged particles.
F is the electrostatic force between charges q₁ is the magnitude of the first charge in Coulombs q₂ is the magnitude of the second charge in Coulombs r is the shortest distance between the charges in m k e is the Coulombs constant. Coulombs Law of Electrostatics. The Coulomb constant the electric force constant or the electrostatic constant denoted k e k or K is a proportionality constant in electrostatics equations.
As a general statement coulombs law force between multiple charges is always exerted radially over a straight line joining the centers of the charged. La distance qui les sépare en mètres. Download Conductors and Insulators Cheat Sheet PDF.
On the Earths surface a mass of 1 kg pushes on its support. Observe how changing the sign and magnitude of the charges and the distance between them affects the electrostatic force. Le coulomb de symbole C est lunité de charge électrique du Système international une des unités dérivées du SI.
13 the magnitude of the force between the two charged spheres is. The force exerted by one charge q on another charge Q is given by Coulombs law. Remarks on Vector Form of Coulombs Law.
Coulombs Law - PhET. This force is too small to cause any visible effect but if you apply the principle of gravitational force to planets or stars its effects will begin to show. Coulombs law is an experimental law that quantifies the amount of force between two stationary electrically charged particles.
Force - Measured in Newton - Force is any interaction that when unopposed will change the motion of an object. In SI base units it is equal to 8987 551 7923 14 10 9 kgm 3 s 4 A 2. TraPPE-UA2 addresses shortcomings of the TraPPE-UA force field by optimization of the molecular shape ie placement of Lennard-Jones sites of the balance between Lennard-Jones and Coulomb interactions and use of the Kong combining rules.
1 N is the force of Earths gravity on a mass of about 102 g. We can think of the forces between charges as something that comes from a property of space. R is the distance between the charges.
Coulombs law states that the electrical force between two charged objects is directly proportional to the product of the quantity of charge on the objects and inversely proportional to the square of the separation distance between the two objects. La force électrostatique qui sapplique entre eux. Now the force on charge q 2 due to q 1 in vector form is.
It exists between all objects even though it may seem ridiculous. Coulombs law is formulated as follows. The electric force between charged bodies at rest is conventionally called electrostatic force or Coulomb force.
Gausss Law cont 8 Kelvin Water Drop Generator. That property is called the electric field. Electrostatic Induction 3 Coulombs Law cont.
Coulombs law describes the amount of electrostatic force between stationary charges. For example while you read these words a tiny force arises between you and the computer screen. We begin with the magnitude of the electrostatic force between two point charges q and Q.
To calculate the Coulombs law forces between multiple charges we use the principle of superposition. The electric force between a stationary charged body is conventionally known as the electrostatic force or Coulombs force. Gausss Law 7 Electric Flux cont.
K has the numerical value of 899 10 9 newtons-square metre per coulomb squared Nm 2 C 2. Whatever Coulomb style is specified in the input command file the short-range Coulombic interactions are computed by this formula modified by an appropriate smoother for the smooth Ewald and PPPM styles. The quantitative expression for the effect of these three variables on electric force is known as Coulombs law.
Remember that force is a vector so when more than one charge exerts a force on another charge the net force on that charge is the vector sum of the individual forces. The bold characters in the equation indicate the vector nature of the force and the unit vector r is a vector that has a size of one and that points from charge Q 2 to charge Q 1The proportionality constant k equals 10 7 c 2 where c is the speed of light in a vacuum.
Coulomb S Law The Electrostatic Force Between Point Charges Is Directly Proportional To The Produ Math Graphic Organizers Solving Equations Math Word Problems

Imp Coulomb S Law Similar To Gravitational Force Equation Physics Lessons Learn Physics Physics And Mathematics

An Awesome Infographic About Coulomb S Law Based On A Tutorial Posted By Student At Mycollegepal Forum For Tutoria Sciences Physiques Culture Generale Science

Coulomb S Law Youtube Physics Law School Hacks

Electric Force And Coulomb S Law Simple Arduino Projects Arduino Arduino Projects

0 Response to "Coulomb Force"
Post a Comment